Laboratory Research Focus Areas:
The role of phosphorylation in regulating the antidiabetic effects of FFA4 (GPR120) |
||
|
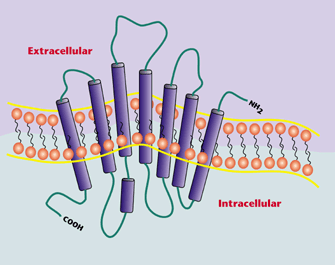
FFA4, formerly referred
to as GPR120, is a recently indentified
unsaturated free-fatty acid receptor that
recognizes long-chained fatty acids,
including the omega-3-fatty
acids a-linolenic acid
(ALA), docosahexaenoic acid (DHA), and
eicosapentaenoic acid (EPA). Agonism
of FFA4 has been shown to promote profound
anti-inflammatory and antidiabetic effects.
Specifically, FFA4 agonism has been linked
to secretion of glucagon-like peptide-1
(GLP-1) and downstream insulin release, and
has also been shown to play major roles in
thwarting insulin resistance, inflammation,
and weight gain. As such, FFA4 has
drawn considerable interest as a target for
treatment of type-2 diabetes and obesity.
|
||
β2-Adrenergic Receptors and Reactive Oxygen Species |
||
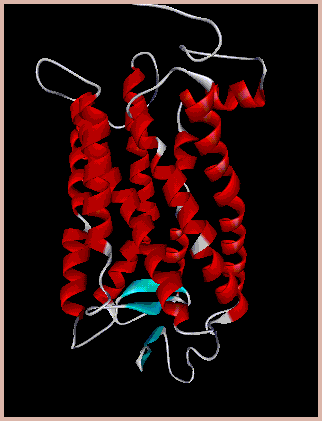
The β2-adrenergic
receptor (β2AR) is one of the best
characterized GPCRs, mediating physiological
responses to epinephrine and norepinephrine.
Many β2-receptor acting agents are utilized
in the clinical therapy of asthma, COPD, and
emphysema. While the effects of
activation of β2-receptors have been studied
in great detail, our laboratory is
interested in a more recently linked aspect
of β2-receptor signaling, namely, generation
of reactive oxygen species (ROS).
|
||
The role of FFA receptors in human health and diseases |
||
We are currently investigating the role of FFA receptors, namely FFA1/GPR40 and FFA4/GPR120, which are agonized by long-chain FFA inlcuding omega-3, omega-6, and omega-9 fatty acids in a variety of human systems, including neurodegenerative diseases, such as Parkinson's Disease, pulmonary diseases such as asthma, as well as in cancers. We are also very interested in the short-chain FFA receptors FFA2/GPR43 and FFA3/GPR41, particularly their roles in mediating the functions of the gut microbiome, which generate SCFA. These projects rely heavily on in vitro pharmacology and molecular biology. |
||
Molecular pharmacology of the drug of abuse xylazine |
||
Illicit use of the surgical anesthetic xylazine has grown dramatically and over the last few years alone, the illicit use of xylazine-adulterated opioids, including fentanyl, has increased exponentially, which has resulted in a variety of public health concerns, including necrosis of the skin. In collaboration with Dr. Clint Canal's laboratory, a recent NIH grant supports a project that seeks to fully characterize the molecular pharmacology of xylazine at α-adrenergic receptors. This research can shed light on the cellular mechanisms of xylazine that may cause skin damage as well as other peripheral effects. |
||
Selective muscarinic acetylcholine receptor drug development |
||
Dr. Moniri and Dr. Canal are co-founders of Channel Therapeutics, LLC, a drug development startup that seeks to discover and develop selective muscarinic acetycholine receptor (MAChR) antagonists for clinical therapy of a variety of disease states including Parkinson's disease, urinary tract and gastrointestinal disorders, and airway disorders. Channel Therapeutics portfolio of patented compounds is being developed to be highly selective for one subtype of MAChR subtype over the other four subtypes, reducing the side effect potential of these novel anticholinergic agents. |
Last Updated: 10/20/2024
Copyright 2025İMercer University
All Rights Reserved
Copyright 2025İMercer University
All Rights Reserved