|
Dr. Moniri’s research
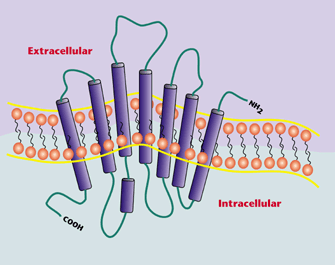
interests focus on pharmacology and biochemistry of G
protein-coupled receptors (GPCRs). These cell-surface
receptors comprise the largest gene family in the human genome and
also represent the largest class of drug targets, accounting
for roughly 40-50% of drugs used clinically today. By coupling
intracellularly to heterotrimeric G proteins, GPCRs are able to
transduce signals from a variety of extracellular stimuli, including
neurotransmitters, hormones, and sensory stimuli.
 |
 |
Dr. Moniri's laboratory currently has two major
research projects. The first is focused on
novel signaling pathways of the
β2-adrenergic
receptor (AR). Our laboratory has shown that agonist-stimulation of
β2AR leads
to generation of intracellular reactive oxygen species (ROS),
formation of which is required for G protein dependent signaling.
We have also recently demonstrated that ROS are capable of feeding
back to oxidize
β2AR cysteine
residues to S-Sulfenic acids, suggesting ROS-mediated
post-translational modification of the receptor. Most
recently, we have demonstrated that ROS are required for
β2AR
interactions with
β-arrestins and
β-arrestin
dependent signaling. We are interested in further
understanding the impact of ROS on
β2AR signaling
in disorders of the lungs,
heart,
and kidneys, which express high densities of
β2AR.
This project relies heavily on in vitro pharmacology and
molecular biology, as well as medicinal chemistry.
The Moniri laboratory is also interested in GPCRs which are attractive targets for pharmacotherapy of
neuro-endocrine disorders. Recent evidence implicates several newly
“deorphanized” GPCRs as important participants in the physiological
regulation of insulin secretion, as well as regulation of caloric
intake and satiety. While the GPCRs involved have been identified
and linked to these downstream physiological functions, the
molecular intracellular pharmacology and receptor biochemistry which
drives endocrine activity remains elusive. Research projects in Dr.
Moniri’s lab are directed towards characterization and elucidation
of intracellular signaling pathways associated with these GPCRs, in
particular, the long chain free-fatty acid receptor, FFA4,
also known as GPR120.
Specifically, FFA4 agonism has been linked to
secretion of glucagon-like peptide-1 (GLP-1) and downstream insulin
release, and has also been shown to play major roles in thwarting
insulin resistance, inflammation, and weight gain, suggesting
importance of this receptor in etiology of type-2 diabetes and
obesity. Our laboratory is interested in FFA4 regulation,
particularly with respect to phosphorylation.
Identification and characterization of potential endogenous or
dietary ligands, as well as development of novel synthetic ligands,
along with characterization of FFA4 biochemistry and intracellular
signaling cascades will provide a mechanistic basis for rationale
drug design to treat disorders such as diabetes and obesity.
Projects will encompass a broad spectrum of biomedical and
pharmaceutical sciences including in vitro and in vivo
pharmacology, molecular biology, biochemistry and medicinal
chemistry.
|
|



